One of the most talked-about natural nutritional ingredients in recent years, ashwagandha’s safety is a top concern for both consumers and formulators. To define its safe usage limits, relevant institutions conducted multi-layered safety assessments on ashwagandha’s core components and extracts—covering everything from cytotoxicity to long-term animal exposure. Below are the key findings and conclusions from these tests.

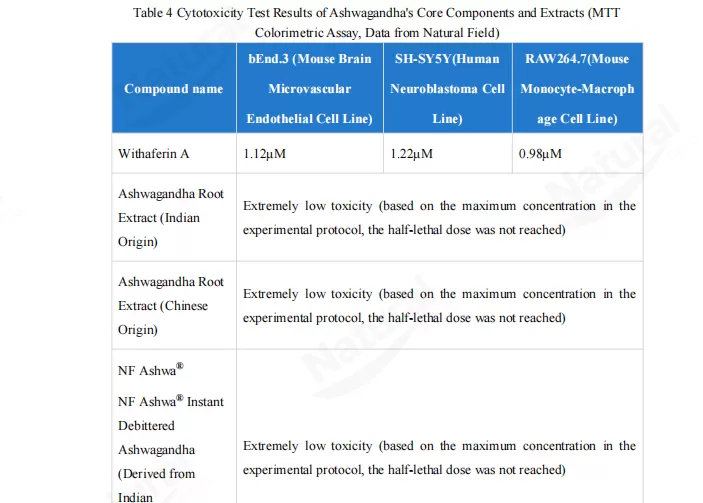
In vitro cytotoxicity testing is a foundational step in raw material safety assessments. For this study, the MTT assay was used to test ashwagandha’s isolated active compound (withaferin A) and three extracts (Indian ashwagandha root extract, Chinese ashwagandha root extract, and NF Ashwa™ water-soluble de-bittered ashwagandha) across 3 representative cell lines (mimicking vascular endothelial, neural, and immune cell environments):
Withaferin A showed some toxicity across all three cell lines, with a half-maximal inhibitory concentration (IC50) ranging from 0.98 to 1.22μM.
By contrast, all three ashwagandha extracts (from different regions and processed forms) exhibited “very low toxicity” within the tested concentration range—they didn’t reach a half-lethal dose even at the highest tested levels.
This result makes it clear: ashwagandha extracts are significantly safer for cellular use than isolated active compounds, so opting for extracts is the more reliable choice in practical applications.
To further confirm in vivo safety, the research team followed Organization for Economic Co-operation and Development (OECD) guidelines to run a series of animal tests—covering genotoxicity, acute/subacute/subchronic toxicity, and reproductive/developmental toxicity:
Genotoxicity: No Mutagenic Risk
Three standard tests validated ashwagandha’s genetic safety:
Bacterial reverse mutation assay: No increase in revertant colonies at doses of 0.156–5.00 mg/plate.
Chromosome aberration assay: No chromosomal damage at concentrations of 0.25–2.00 mg/ml.
In vivo micronucleus assay: No increase in micronucleated cell frequency (MNCE) or bone marrow toxicity in mice at doses of 500–2000 mg/kg.
Conclusion: Ashwagandha shows no genotoxicity within the tested ranges.
Acute Oral Toxicity: Low Risk, High Tolerance
Using female Wistar rats as the model, ashwagandha was administered orally at doses of 500, 1000, and 2000 mg/kg body weight, with 15 days of observation:
No animal deaths, illness, or abnormal clinical signs were observed. Body weights increased normally, and no organ pathology was found upon dissection.
The median lethal dose (LD50) cutoff exceeded 5000 mg/kg body weight. Per GHS classification, this falls into Category 5 or is unclassified—meaning there’s no acute oral toxicity risk.
Subacute/Subchronic Toxicity: Safe for Long-Term Use
Subacute (28-day): Wistar rats (30 male/30 female) received daily oral doses of 200–800 mg/kg body weight of ashwagandha root extract. Results: All groups had normal weight gain and food intake; hematological and biochemical markers stayed within normal ranges (only minor elevations in some markers in the high-dose group, which still met industry standards). Markers returned to normal in the satellite group (observed for 43 days).
Subchronic (90-day): SD rats received daily oral doses of 100–1000 mg/kg body weight of ashwagandha. Results: No toxic symptoms or deaths; weight/food intake matched the control group; hematology, biochemistry, organ weights, and histopathology showed no abnormalities. The No-Observed-Adverse-Effect Level (NOAEL) was 1000 mg/kg body weight.
Reproductive & Developmental Toxicity: No Impact on Reproduction
Following OECD guidelines, Wistar rats (40 male/52 female) received oral ashwagandha doses of 500–2000 mg/kg body weight (covering pre-pregnancy and lactation periods):
Parent body weights, reproductive organ weights, and thyroid hormone levels showed no differences from the control group (no dose-dependent abnormalities).
Pup litter size, survival rate, and body weight matched the control group; no external or pathological malformations were seen.
At the highest dose (2000 mg/kg body weight), the parent pregnancy rate dropped slightly (no significant toxicological meaning), and no reproductive organ pathology was found.
Conclusion: Ashwagandha has no adverse effects on parental reproductive function or offspring growth/development.
Combining in vitro and in vivo results, ashwagandha extract safety is fully validated: Whether used short-term, long-term (90 days), or in reproductive/developmental contexts, it shows no significant toxicity—even at doses far higher than the typical human daily recommendation (no more than 100 mg/kg per single intake, or ~21g daily).
This conclusion provides a reliable safety basis for ashwagandha’s use in functional foods, dietary supplements, and similar products.
As a specialized supplier with 20 years of experience in nutritional raw materials (founded in 2005), Natural Field’s ashwagandha extracts are core products developed and manufactured to these strict safety standards. We not only released the Ashwagandha White Paper in 2025 (systematically organizing its safety and efficacy data) but also adhere to the principle of “producing nutritional ingredients with pharmaceutical-grade standards.” Backed by a 32-person professional R&D team, 39 national patents, and nearly 10 domestic/international certifications (including FSSC 22000 and ISO), we ensure the purity and stability of every batch of ashwagandha extract.
Today, our ashwagandha products serve global leading brands like Nestle and LG, reaching 86 countries and regions. With strategic warehouses in the U.S., UK, and more, we deliver stable, efficient supply chains. If you need compliant, high-safety ashwagandha raw materials or customized nutritional formulation solutions, reach out to us—Natural Field’s expert technical support and flexible services will help bring your wellness products to market.