In the realm of the intestinal microbiome, Akkermansia muciniphila (Akk bacteria for short) has long been celebrated as a "star probiotic." However, a recent study by a research team from Tsinghua University, published in Nature Microbiology, has delivered a stunning "role reversal" for this so-called "intestinal guardian"—revealing that under specific immune conditions, the seemingly harmless Akk bacteria can transform into an accomplice of pathogens, covertly exacerbating the escalation of intestinal infections. How does this "double agent" drama unfold in the microbial world?

The name Akkermansia muciniphila—or "Akk bacteria" for short—already hints at its unique survival strategy: this "mucin-loving" microbe relies primarily on mucin, the main component of the intestinal mucus layer, as its food source. Far from being a gut disruptor, this "mucus eater" acts as a dedicated intestinal caretaker, playing multiple pivotal roles in maintaining digestive wellness:
By breaking down mucin, Akk bacteria continuously stimulate intestinal epithelial cells to repair themselves and secrete more mucus, actively rebuilding and maintaining an optimal intestinal mucosal barrier—a protective layer that shields the gut from harmful invaders.
Through metabolizing mucin, it produces beneficial byproducts like butyrate, a short-chain fatty acid that serves as a direct energy source for intestinal cells. These metabolites also help regulate the body’s energy metabolism, linking gut health to overall metabolic efficiency.
Akk bacteria communicate directly with immune cells, fine-tuning the intensity of inflammatory responses. This interaction ensures a delicate equilibrium in the gut’s local immune system, preventing both excessive inflammation and immune neglect.
By reinforcing the physical mucus barrier, Akk bacteria create a robust shield that blocks toxins and pathogens from penetrating the intestinal lining and entering the bloodstream, enhancing the gut’s first line of defense.
This multifunctional role—nourishing the mucus layer, repairing the gut, regulating immunity, and supporting metabolism—has solidified Akk bacteria’s long-standing status as a "star probiotic" and an iconic symbol of gut health.
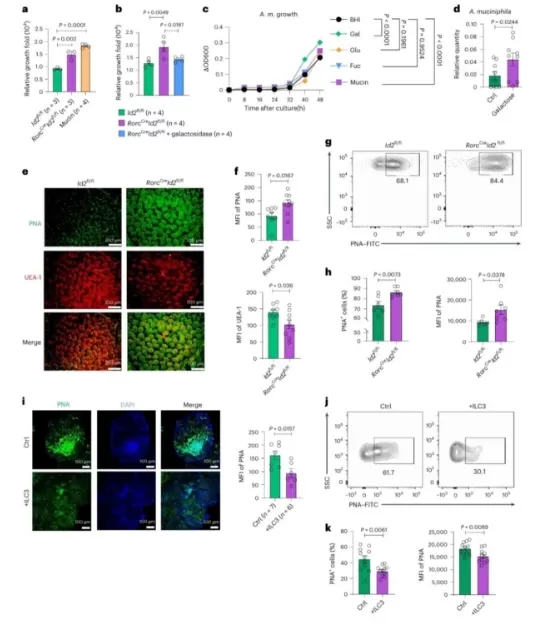
The story took a dramatic turn when researchers observed unusually severe intestinal infections in a model lacking ILC3 immune cells—a paradox that uncovered a hidden dark side of Akk bacteria.
In a healthy gut, ILC3 immune cells act as "intestinal patrol guards," continuously secreting the IL-22 cytokine. This molecule acts like a protective coating for intestinal epithelial cells, performing two key functions:
· A. Physical Barrier Reinforcement: By increasing fucosylation, it creates a smooth mucosal surface that directly blocks pathogenic bacteria from adhering to the gut lining.
· B. Metabolic Control: It precisely regulates galactosylation levels, limiting the carbon sources available for excessive Akk bacteria growth—keeping their population in check.
Without ILC3 cells, this finely tuned system collapses, triggering a dangerous cascade:
1. Uncontrolled Proliferation: Galactosylation in intestinal cells surges, providing Akk bacteria with an "unlimited buffet" of nutrients. This causes their population to explode exponentially.
2. Toxic Metabolite Production: The overgrown Akk bacteria start flooding the gut with succinate, a short-chain fatty acid that turns harmful at high levels:
· A. Microenvironment Alteration: It lowers gut pH, creating an ideal environment for pathogens like Citrobacter rodentium to colonize and multiply.
· B. Pathogen Activation: Succinate directly switches on pathogenic genes, including virulence factors Tir (adhesin) and Ler (pathogenicity island regulator). This boosts the pathogens’ ability to stick to intestinal cells and secrete toxins.
This "good intention gone wrong" chain reaction transforms Akk bacteria from a gut protector into a silent accomplice of pathogens, exponentially amplifying infection severity and risk.
This study unveils for the first time the intricate mechanism governing the dynamic equilibrium between the host immune system and gut microbiota—a delicate dance that determines whether bacteria act as allies or adversaries.
ILC3 immune cells → Secrete IL-22 cytokine → Inhibit gut cell galactosylation → Restrict excessive Akk bacteria growth → Preserve mucosal layer stability
ILC3 deficiency → Insufficient IL-22 → Uncontrolled galactosylation → Explosive Akk bacteria proliferation → Excessive succinate secretion → Enhanced pathogen virulence
This discovery shatters the conventional belief that "probiotics are universally beneficial", proving that a microbe’s role hinges entirely on the host’s immune context. Like species in an ecosystem, unregulated dominance of a single bacterial strain—even a "beneficial" one—can tip the scales, disrupting the gut’s overall homeostasis.
ILC3s Regulate Gut Glycosylation to Restrict Akk Bacteria Proliferation
This research does not diminish Akk bacteria’s probiotic potential but serves as a crucial reminder: true gut wellness lies in understanding the context-dependent nature of our microbial allies. Here’s what it means for managing gut health:
Maintaining the normal function of immune cells like ILC3—which produce the protective cytokine IL-22—may be more critical than simply supplementing probiotics. A robust immune system acts as the "conductor" of gut microbiota, ensuring beneficial bacteria like Akk stay in their protective role rather than shifting to a harmful state.
Gut bacteria don’t operate in a vacuum—their effects depend on your unique immune environment. Routine assessments of immune markers (e.g., IL-22 levels) alongside microbiome profiling could help tailor interventions: what boosts gut health for one person might backfire in someone with immune dysfunction.
Gut health is a tripartite collaboration between immune cells, intestinal epithelial cells, and microbiota. Disruptions in any component—whether a dip in ILC3 activity, a weakened mucosal barrier, or unchecked bacterial overgrowth—can trigger cascading issues. Nurturing this delicate ecosystem requires a holistic approach, not just targeting single microbes.
As we rethink the "dual-natured probiotic" that is Akk bacteria, the lesson is clear: true gut health starts with respecting the complexity of this inner ecosystem. Instead of chasing "one-size-fits-all" solutions, prioritizing immune resilience—the body’s own ability to regulate microbial interactions—may be the key to fostering harmonious coexistence between our cells and their microbial partners.
How does your gut microbiome converse with your immune system? Follow Natural Field, a professional immune support ingredients manufacturer, for the latest cutting-edge gut health research and join us in unraveling the mysteries of the human microbiome!