As demand for NMN-based products continues to grow, raw material selection has become a critical decision point for supplement brands, contract manufacturers, and formulation teams. Beyond purity and specification, buyers increasingly evaluate delivery efficiency, stability, and application flexibility—areas where liposomal NMN offers clear advantages over conventional NMN powder.
This article outlines the practical benefits of liposomal NMN from a procurement and formulation perspective, helping buyers assess whether it fits their product strategy.

Standard NMN powder is widely used, but in certain applications it presents challenges that buyers should consider:
Sensitivity to moisture and processing conditions
Stability concerns in liquid or multi-ingredient formulations
Limited differentiation in increasingly competitive NMN markets
For brands targeting premium positioning or advanced delivery claims, these limitations often prompt a shift toward encapsulation-based solutions.
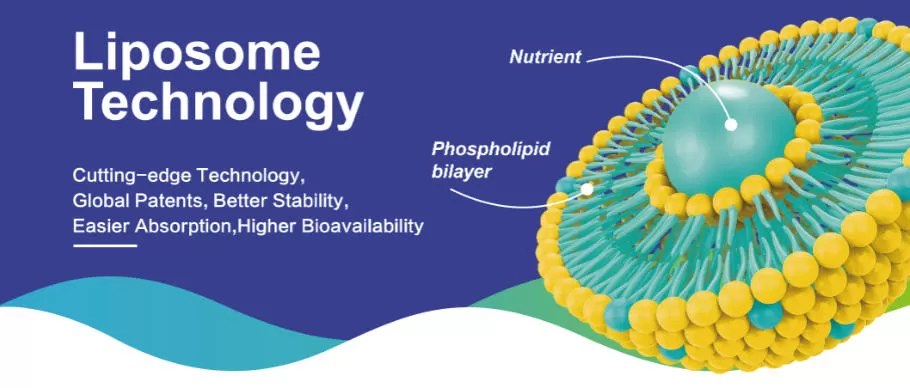
Liposomal NMN is produced by encapsulating NMN within phospholipid bilayers, creating micro-scale vesicles that protect the active ingredient and support improved delivery behavior.
From a procurement standpoint, this technology is not only about innovation—it directly impacts product performance, shelf stability, and formulation options.
Liposomal encapsulation helps protect NMN from environmental stress during processing and storage. This is especially valuable for:
Liquid supplements and sachets
Functional beverages
Multi-active formulations
For buyers, this translates into lower risk of degradation and more predictable product performance.
While conventional NMN powder relies on standard absorption pathways, liposomal delivery systems are designed to improve interaction with biological membranes.
For brands, this supports:
Clear differentiation in product positioning
Justification for premium pricing strategies
Stronger technical narratives for marketing and education
From a sourcing perspective, liposomal NMN allows greater formulation freedom across:
Liquids and oral sprays
Sachets and stick packs
Advanced capsule or combination products
This flexibility is particularly important for buyers developing next-generation dosage forms.
In competitive NMN markets, liposomal formats help move products away from commodity positioning. For procurement teams, this means sourcing an ingredient that supports higher-margin SKUs rather than price-driven competition.
When selecting a liposomal NMN supplier, buyers should assess:
NMN purity and specification transparency
Encapsulation efficiency and consistency
Batch-to-batch stability data
Scalability and long-term supply capability
Technical documentation and formulation support
A reliable supplier should be able to provide clear specifications, application guidance, and quality control standards.
Natural Field supplies both high-purity NMN powder and liposomal NMN designed for professional nutraceutical and functional product development.
From a buyer’s perspective, our liposomal NMN solutions offer:
Controlled specifications and consistent quality
Scalable production for commercial volumes
Application support across multiple formulation types
Whether you are evaluating liposomal NMN for product differentiation, formulation stability, or premium market positioning, Natural Field can support your sourcing decision with reliable raw material options.
To request specifications, technical data sheets, or samples of liposomal NMN, please visit NaturalFieldInc.com or contact our team directly.