
Abstract: In a high-quality study published in May 2025, a research team from the Department of Nutrition at the Naval Medical University of the People's Liberation Army designed a novel "screening system"—using a luciferase reporter model to "monitor" the activity of miR-122, akin to installing a "signal light" for miR-122. When a compound enhances miR-122 activity, the "signal light" brightens. Subsequently, they conducted large-scale high-throughput screening of 2,543 natural compounds, effectively searching for the "ideal partners" for miR-122.
After rigorous screening, dihydroberberine emerged as the standout candidate among 2,543 natural compounds: it not only significantly upregulates miR-122 levels but also exhibits no cellular toxicity—demonstrating both efficacy and safety.
In a high-iron-diet-induced mouse model of MASLD (Metabolic Dysfunction-Associated Steatotic Liver Disease), dihydroberberine demonstrated remarkable liver-protective effects:
Restores miR-122 Levels: Reverses iron-overload-induced reduction of miR-122, effectively reactivating the liver's "regulator".
Improves Lipid Metabolism: Reduces hepatic triglycerides and total fat content, leading to a visibly "cleaner" liver (evidenced by Oil Red O staining).
Prevents Oxidative Damage: Lowers levels of lipid peroxidation markers (e.g., MDA, LPO), protecting hepatocyte membranes from degradation.
Mitigates Liver Injury and Fibrosis: Significantly decreases ALT and AST (key liver injury markers) and alleviates hepatic fibrosis progression.
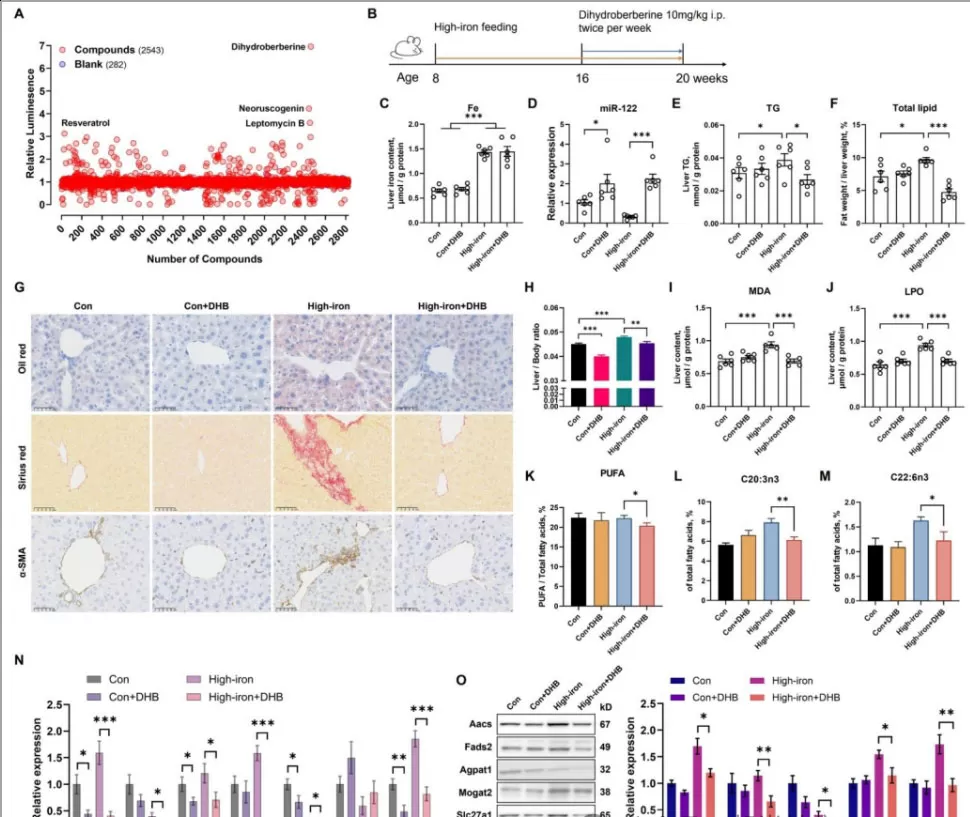
Figure D: miR-122 regulation – Iron overload caused a sharp decline in miR-122 expression, while DHB treatment restored it to near-normal levels.
Figures E-H: Lipid metabolism improvement – Iron overload induced hepatic triglyceride and total lipid accumulation, which were reduced following DHB administration.
Figures I-J: Oxidative damage mitigation – Iron overload increased oxidative products (MDA, LPO, etc.), while DHB treatment significantly decreased these markers.
Figures K-M: Fatty acid balance restoration – DHB treatment reversed iron overload-induced dysregulation of polyunsaturated fatty acids and restored beneficial fatty acid ratios.
Figures N-O: Protein regulation – Iron overload disrupted the expression of lipogenesis-related proteins, which was normalized by DHB intervention.
Among 2,543 compounds screened, dihydroberberine (DHB) stood out not by chance, but by demonstrating both the strongest miR-122 upregulation and perfect cellular biocompatibility—a critical foundation for clinical translation.
Iron overload damages the liver through a defined cascade:
Iron overload → miR-122 crash → dysregulated lipogenic proteins → lipid accumulation + oxidative stress → exacerbated liver disease.
DHB interrupts this vicious cycle by:
DHB supplementation → miR-122 recovery → suppressed lipogenic proteins → reduced lipid deposition + mitigated oxidative damage → hepatic "cooling down".
The study conclusively demonstrates that DHB:
Upregulates miR-122 to silence detrimental genes (e.g., Aacs, Fads2, CYPs) driving lipogenesis and oxidation
Fundamentally breaks the "iron overload → metabolic dysfunction" feedback loop
Offers dual action—metabolic regulation and hepatoprotection—for populations suffering from iron overload and MASLD
From elucidating how iron and miR-122 synergistically harm the liver, to identifying DHB through systematic screening, this research illuminates a promising therapeutic avenue for MASLD intervention.
References:
Yuxiao Tang 1, Zelong Gao et al. Breaking the synergism of iron overload and miR-122 to rescue lipid accumulation and peroxidation in MASLD. Pharmacol Res. 2025 May:215:107728.