When oral vitamin C delivers less than 20% bioavailability while liposomal encapsulation boosts it beyond 90%, this drug-delivery-born technology has fundamentally redefined the game rules for nutraceuticals and functional skincare. As a liposomal manufacturer with proven expertise in liposome raw material production, Natural Field decodes the scientific decision chain behind industrial-scale success—from phospholipid screening and process parameters to precision control of encapsulation efficiency.
Phospholipid Type | Raw Material Cost (USD/kg) | Encapsulation Efficiency Range | Stability (25℃/12 months) | Suitable Actives |
Soybean Lecithin | $80-120 | 60-75% | Degradation rate ≤8% | Hydrophilic compounds (Vitamin C, Glutathione) |
Sunflower Lecithin | $150-200 | 75-92% | Degradation rate ≤3% | Sensitive compounds (NMN, Polyphenols) |
Hydrogenated Lecithin | $300-450 | 85-95% | Degradation rate ≤1.5% | High-value pharmaceuticals (Paclitaxel) |
Conclusion: Sunflower lecithin emerges as the cost-effective option for sports supplements and anti-aging products, owing to its hypoallergenic properties (non-GMO) and superior oxidative stability (iodine value <80).
Warning threshold: Lysophospholipid content >2% induces lipid bilayer rupture
Solution: Implement HPLC-ELSD detection to guarantee phospholipid purity ≥98%
Manufacturing Processes: How Microfluidic Technology Overcomes Encapsulation Efficiency Barriers
Parameter | Thin-Film Dispersion | Microfluidic Technology | Industrial Advantage |
Particle Size Control | 100-500nm (high variability) | 50-150nm (±10nm) | Avoids hepatic first-pass effect |
Encapsulation Efficiency | 40-65% | 75-95% | Reduces active ingredient waste by >30% |
Batch Consistency | CV >15% | CV <5% | Meets core requirements for GMP certification |
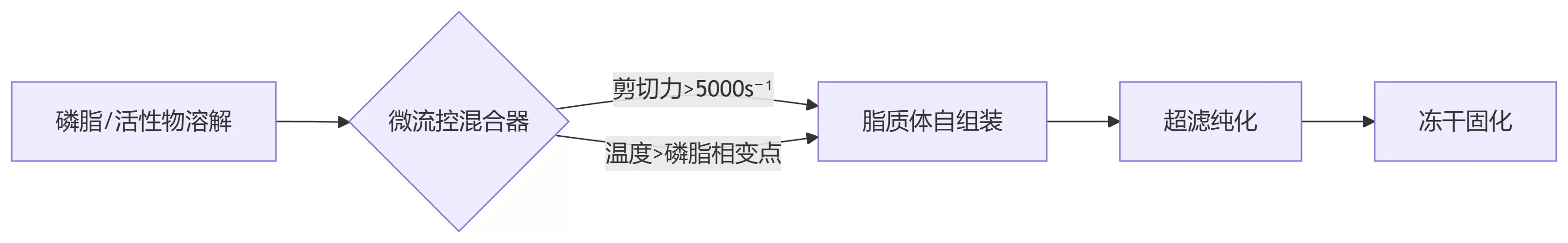
Critical Control Points:
1. Temperature Trap:
Soy lecithin phase transition at -20℃ vs. Sunflower lecithin at 55℃ — ±1℃ deviation causes 12% encapsulation efficiency loss
2. Shear Stress Failure:
Exceeding critical thresholds (e.g., 8000s⁻¹ for soybean lecithin) induces catastrophic lipid bilayer rupture
Method | Cost per Sample | Time Required | Margin of Error | Application Scenario |
Dialysis | $20/sample | 24h | ±25% | Preliminary R&D screening |
Ultracentrifugation | $50/sample | 3h | ±15% | Small-batch production |
HPLC-MS | $150/sample | 1.5h | ±2% | GMP-certified batch release |
Industry Misconception:
Encapsulation Efficiency (%) = (Encapsulated active / Total active) × 100%
Critical Flaw: Fails to deduct free active adsorbed on liposome surface!
Corrected Formula: *True EE (%) = [1 - (Free active / Total active)] × 100%*
Validation Required: Ultrafiltration-MS hyphenated technique
When selecting raw material suppliers, rigorously verify these three capabilities:
Phospholipid Traceability System: Farm-to-lab documentation for non-GMO sunflower lecithin
In-process Monitoring: Real-time temperature/shear force feedback in microfluidic systems
Testing Method Transparency: Provision of raw HPLC-MS chromatograms with full calculation logic
Natural Field as a specialized supplier focused on liposome technology R&D and production, we leverage our in-house manufacturing facilities' robust capacity and stringent quality control to deliver high-stability, high-bioavailability liposome solutions for global clients. Every liposome product we develop leads the industry in encapsulation efficiency, stability, and functionality. Through technological empowerment of products and professional commitment to quality, we look forward to partnering with you to co-create the future value of liposome applications.